CHEM 558 - Electrochemistry – Syllabus – Fall
2024
Frank Cheng,
Renfrew 003,
Office Hours:
Text: Understanding Voltammetry, 2nd
Ed. Richard G. Compton, Craig E. Banks
This course will also use web links and
handout lecture notes.
Electrochemistry Basics*Cambridge
University Lecture Notes*MIT
1.0
Equilibrium Electrochemistry – Chapter 1 (Do
Homework Set 1, Review Problems from Chem 454)
1.1
Nernst Equation
Reference Electrodes (SHE, SCE, Ag/AgCl)
Review * Daniell
Cell
Standard Electrode Potentials
Connection between Ecell, ∆G,
and K
Single Electrode Potentials
Potentials
for sums of half reactions
1.3
Advanced Aspects of Nernst Equation (Lecture Notes
J. Phys. Chem. Ref. Data. 10,
1981, 1175)
1.5 Pourbaix Diagrams
1.6 Special Topics – ZVI based remediation of
halocarbons, TCE
(Reactive Iron Barrier, link
2, 3)
Video
2.0 Electrode Kinetics
2.1 Introduction
2.2 Butler–Volmer
Kinetic (Text: Chapter 3.3 – 3.5
2.3 Tafel
Analysis (University of Cambridge, Example of Corrosion Inhibition, Gamry.com
Corrosion of Alaska Oil Pipeline, NYT
2.4 Marcus Theory
2.5 Special Topic - Redox
Flow Batteries, improvement of kinetics.
3.0 Diffusion and Controlled
Potential Methods
3.1 Mass Transport
– Diffusion, Migration and Convection
3.2 Introduction
3.3 Potential Step
– Diffusion Control
3.4
Potential Step – Kinetic Control
3.5
Chronocoulometry
3.6 Pulse
Methods – Chapter 9 (Libretext)
Polarography
Pulse
Polarography/Voltammetry
Stripping Analysis
4.0 Cyclic Voltammetry – Chapter 4 Practical Guide
4.1 Introduction Wikipedia,
Anal. Chem. 36:706-23, 1964., Wolfram Research Demo, Irving Shain Obituary.
New – Power buffering. Vanadium Redox Flow
Battery. Measurement of electrode
kinetics by cyclic voltammetry.
4.1.1 Radial vs Semi-infinite liner
diffusion. Example
of both contributing to the CV.
4.1.2 Osteryoung’s Equation.
“Linear sweep voltammetry at very small stationary disk
electrodes. Aoki, Koichi; Akimoto, Koji; Tokuda, Koichi;
Matsuda, Hiroaki; Osteryoung, Janet.
Tokyo Inst. Technol., Grad. Sch.
Nagatsuta, Yokohama, Japan.
Journal of Electroanalytical Chemistry and Interfacial
Electrochemistry (1984), Vol. 171, p219”
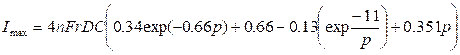
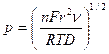
4.2 Emphasis on Coupled Homogeneous Reactions
A study of an EC’ Mechanism from my research group,
Digisim
in J. Chem. Ed. Digielch
A synthetic
chemist's guide to electroanalytical tools for studying reaction mechanisms Chem Sci. 2019 Jul 14; 10(26): 6404–6422
Literature – A manual for
modeling
Nicolson’s
Paper, Analytical Chemistry, 37, 1965, 1351
A Review
Article: Chem. Sci., 2019, 10, 6404-6422
An example of
a CE mechanism: Organometallics, 1999,
18 (10), pp 1854–1861
EC Mechanism
CV of Dopamine, Libretext
ECE mechanism
J. Phys. Chem. 76, 1972, 2439. Anal. Chem. 37, 1965, 190.
EC’
Mechanisms J. Phys Chem C. 2011, 115, 11204, PNAS
2017, 114, 11303.
Oxygen
Activation : Ind. Eng. Chem. Res.
2003, 42, 21, 5024–5030
ENVIRONMENTAL SCIENCE &
TECHNOLOGY / VOL. 39, NO. 18, 2005
Ind. Eng. Chem. Res. 2008,
47, 6502–6508
Journal
of Electroanalytical Chemistry 608 (2007) 111–116
4.3
Adsorbed Species https://pubs.acs.org/doi/pdf/10.1021/acs.jpcc.8b05484
4.4 Thin-Layer
Cells Wolfram Research Demo
Lit
Example https://pubs.acs.org/doi/10.1021/acs.analchem.1c04467
5.0 Hydrodynamic Methods
The
rotating disk electrode. Video, Video2
Levich
equation and behavior. Video
Mathematical models at Wolfram Research
RDE Levich Equation
RDE
Ox + ne- = Red
Special
Topic – analysis of fuel efficiencies.
6.0 Recent Topics
6.1 Carbon
Electrodes: Glassy Carbon, Graphene, Graphite, and GUITAR Electrodes
Electron
Transfer at the Basal vs. Edge Planes
J. Phys. Chem C, 2019, 123,
11677
Raman
spectroscopy of carbon
Potential
Windows
6.2 Batteries (An
Overview of Batteries from Powerstream)
Li ion
Lead-Acid
Redox Flow
Batteries
6.3 Fuel Cells
7.0 AC
Techniques
Exam
Schedule
- September 11 – 100 points
- October 9 – 100 points
- November 22 – 100 points
- December TBA, Final – 200 points